Interventional Radiology: Targeting hard-to-treat tumors

As an interventional radiologist, Rahmi Oklu, M.D., Ph.D. (RD ’15), is well aware of the fallibility of medicine.
“When you start practicing as a physician, you begin to see firsthand that so many of the procedures and devices we use aren’t perfect,” he says. “You see patients suffer from complications and outcomes you didn’t expect. There are moments where you wish you could have done more. It stays with you. And it drives you to search for something better.”
This reality hit Dr. Oklu hard when a patient in his 40s came to the hospital with acute bleeding.
“It was a young patient. We did everything we possibly could,” says Dr. Oklu. “The best medicine could not save this patient. The best research, the best hospital care could not save this patient.
“That shook me. If the ‘best’ we had wasn’t enough, then the ‘best’ had to get better.”
The patient’s death inspired Dr. Oklu to think outside the box. What followed was a journey that led to multiple National Institutes of Health Research Project (R01) and Small Business Innovation Research (SBIR) grants, five patents, the founding of a startup company, a clinical trial and an arduous path to U.S. Food and Drug Administration (FDA) clearance — all to put a new embolic product known as Obsidio in the hands of physicians around the U.S. Nationally launched in 2024, Obsidio has already been used over 6,000 times and saved numerous lives by stopping life-threatening bleeding.
Even after this exhausting journey, Dr. Oklu isn’t slowing down. He’s now leading a clinical trial and working toward FDA and European regulatory approvals for a new kind of cancer treatment named inTumo. As a Clinician Investigator and the founding director of the Patient Inspired Engineering Laboratory at Mayo Clinic in Arizona, Dr. Oklu remains at the forefront of medical innovation — turning bold ideas into real solutions that bridge medicine and engineering.
“In medicine, you help people one at a time, and that’s incredibly meaningful,” he says. “But when you take an idea, build a startup and bring that innovation into clinical practice, you’re creating something that can help thousands, even millions. It’s a different kind of impact — one that reaches across the globe.”

A NEW KIND OF TUMOR ABLATION
Dr. Oklu is a leading interventional radiologist specializing in interventional oncology. He’s driven by a mission to transform patient care through minimally invasive, image-guided therapies.
“Dr. Rahmi Oklu is widely recognized for his unique ‘patient-inspired engineering’ approach, where challenging clinical problems drive his research to develop groundbreaking medical devices and biomaterials,” says Joseph Hoxworth, M.D. (RD ’07), chair of the Department of Radiology at Mayo Clinic in Arizona. “His inventions have significantly improved clinical procedures, enabling faster, more effective treatments and positively influencing patient outcomes across various conditions.”
Today, Dr. Oklu is spearheading the development of a revolutionary liver cancer treatment based on an injectable biomaterial known as inTumo. As president and CEO of inTumo Therapeutics, Dr. Oklu has an unwavering focus on securing regulatory approval for this product, and he’s determined to deliver the potentially life-saving technology to patients worldwide.
For now, inTumo is focusing on patients with hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma. These cancers are often unresectable due to underlying disease, tumor location or patient comorbidities. For select patients, liver transplantation offers the best chance of cure. However, staying within transplant eligibility while awaiting a donor organ is challenging.
To help maintain these patients within the transplant criteria, interventional radiologists may perform locoregional therapies (LRTs) such as ablation. But ablation may not be viable if the tumor is large, too close to the pancreas or other important structures, or if comorbidities preclude general anesthesia. Alternatively, some researchers have tried injecting various therapies such as oncolytic viruses, immunotherapy and chemotherapy directly into liver tumors, aiming to deliver a high concentration of the drugs and avoid systemic effects. However, these approaches are still experimental and often fail to achieve uniform distribution or sustained retention within the tumor, limiting therapeutic impact.
Given these limitations, researchers around the world continue to work to expand treatment options. InTumo represents one LRT procedure with great promise for those with liver cancer.
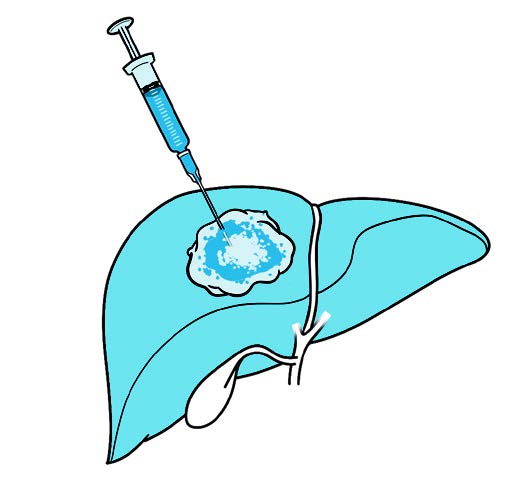
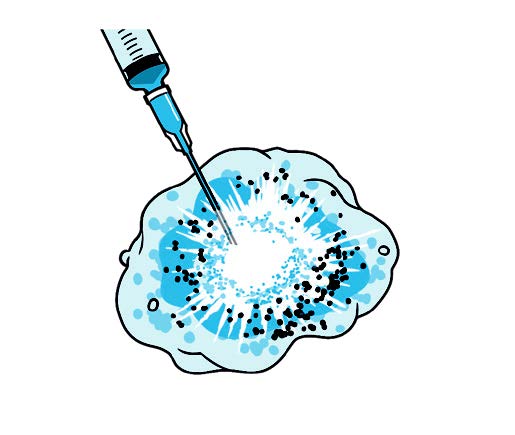
InTumo’s therapeutic approach is simpler, easier and faster than current standard medical therapy, Dr. Oklu says. InTumo is a super-concentrated “designer salt” solution comprised of a proprietary formulation that is percutaneously injected into a tumor and, through a physical process, rapidly pulls the water out of the cancer cells, causing instant catastrophic cell death.
“Picture a tornado; all the fluid gets pulled out of the cell, rupturing the cell membrane,” Dr. Oklu says. “So the mechanism of action is physical force.”
Dr. Oklu and his team first tested the formulation in small and large animal tumor models, then progressed to testing in resected human cancer tissue. They found inTumo was effective in a variety of tumor types and published their results in peer-reviewed publications, including cover articles in Science Translational Medicine and Advanced Materials.
With successful animal studies completed and the formulation enhanced to achieve human-grade quality, inTumo advanced to human testing in a clinical trial (ClinicalTrials.gov ID NCT06689670). In the trial, image guidance is used to inject the substance into liver tumors, often under mild sedation and/or local anesthesia. Dr. Oklu and his team have shown that most of this solution flows out from the injected site into, and out of, the blood stream within several hours. An MRI taken as part of the clinical trial immediately after the procedure is used to determine whether the injection successfully ablated the tumor.
“The beauty of this technology is its speed. Within minutes we know that it has done what it’s intended to do,” he says.
As a physician who treats liver tumors at Mayo Clinic, Dr. Oklu understands firsthand the limitations of current treatments. InTumo, he says, represents a new standard in the ablation of solid tumors.
How it works: Ionic tumor ablation
“EVERYTHING IS ABOUT THE PATIENT”
Dr. Oklu was able to witness the life-changing potential of inTumo when his team was contacted by a mother desperate for help for her 24-year-old daughter.
Diagnosed with cholangiocarcinoma, the daughter had undergone a left lobe liver resection. A follow-up surveillance MRI showed that the tumor had recurred, and despite multiple rounds of chemotherapy, her tumor continued to grow. The mother found her way to Dr. Oklu’s clinical trial, hoping it could deliver a miracle for her daughter.
The patient’s tumor had rapidly grown to 6 centimeters in size, which is substantially larger than tumors typically considered ablatable via percutaneous approaches. But Dr. Oklu decided to include the patient in the trial anyway.
“At the end of the day, I’m a Mayo physician. Everything is about the patient and that’s all that matters,” he says. “I said, ‘If it’s not going to help you, then why am I here? What’s the sense of pushing this technology?’”
An interventional radiologist from Dr. Oklu’s team injected inTumo into the patient’s liver tumor, a procedure that took a matter of minutes. An MRI performed directly after the procedure showed that the approach had substantially ablated the tumor, and the tumor continued to shrink over the next month.
“When I showed her post-treatment scans to the surgical team, they were in disbelief,” Dr. Oklu says. “We had successfully down-staged her tumor to the point where surgery became an option — and she underwent a curative resection. Now, nine months later, she continues to be cancer-free … and is engaged to be married. We didn’t just treat a tumor — we gave her a future.”
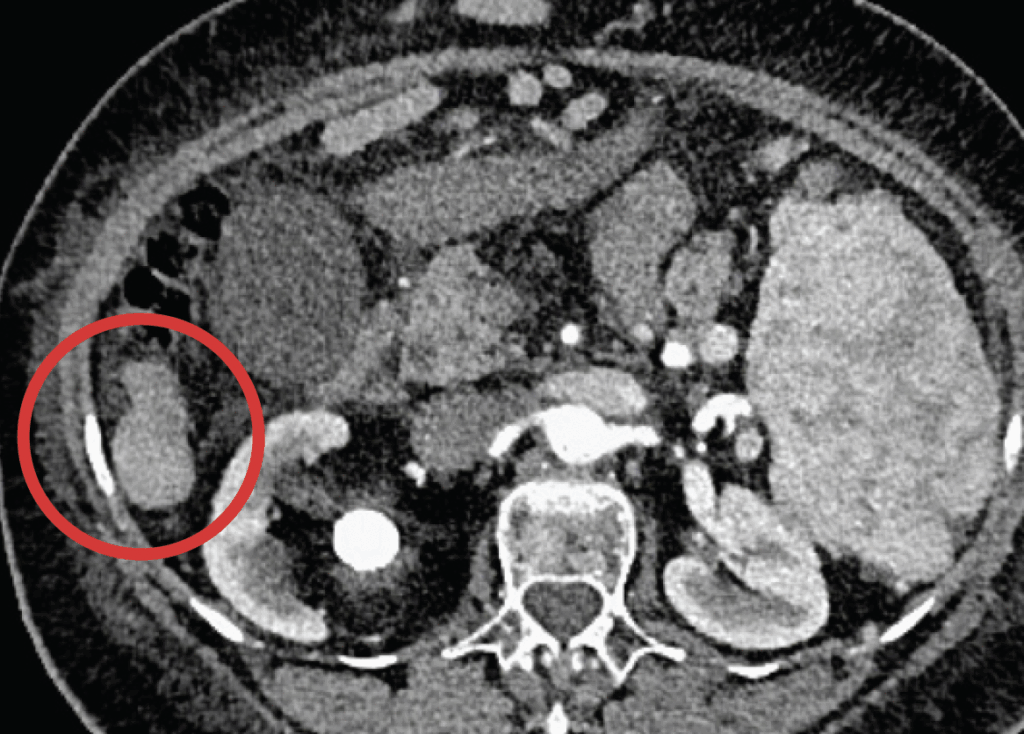
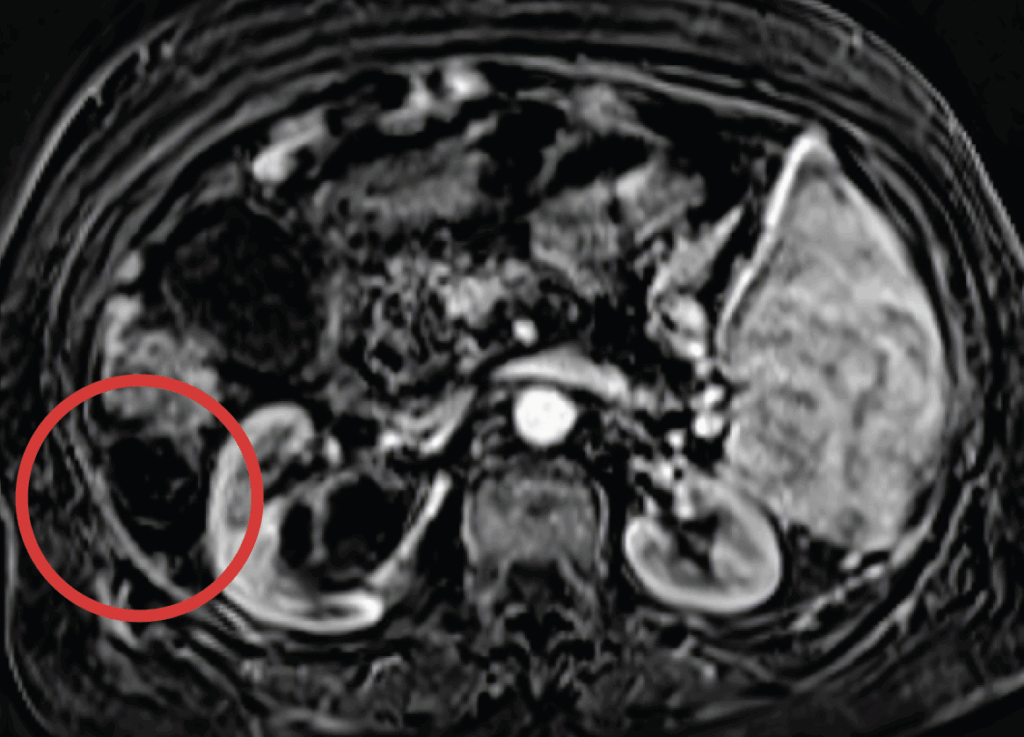
These images show an example of a patient response to inTumo. The top image shows a hepatocellular carcinoma measuring approximately 4 centimeters. This tumor would have been challenging to ablate via microwave because it extends outside the surface of the liver (known as an exophytic tumor). Using ultrasound guidance, inTumo was injected into the tumor via syringe. This led to instantaneous death of the tumor as shown in the bottom image, a contrast-enhanced MRI showing absence of viability of the tumor taken immediately after treatment. The total procedure time was about five minutes and was performed using local anesthesia and mild sedation.
Unfortunately, Dr. Oklu knows that these types of tumors tend to come back. In the case of this patient, he hopes inTumo will be cleared for use to help treat any future recurrence.
Nearly a dozen other clinical trial participants have also benefited from the inTumo formulation. While not curative, inTumo could play an important role in extending life and maintaining transplant eligibility, providing new hope to patients with liver cancer.
And while the ablative properties of inTumo are promising, they represent just one of its potential mechanisms to treat cancer. Dr. Oklu’s team has conducted preclinical animal studies in organs such as the lungs, brain, breast and kidney to explore other potential avenues, including pairing inTumo with chemotherapy, immunotherapy or nanoparticles, and formulating the liquid as a catheter-directed embolic to cut off tumor blood supply and achieve chemical segmentectomy.
Clearly, Dr. Oklu still has plenty to explore. He’ll keep innovating at the bench and working tirelessly to bring these innovations to the bedside. His career is a reminder that innovation is personal; each new idea started with a patient who couldn’t wait for progress.
“Bringing an idea from the lab to the clinic and seeing it improve patients’ lives is one of the greatest rewards in science and medicine,” says Dr. Oklu.
READ THE ENTIRE BENCH TO BESIDE SERIES
This story appears in the latest issue of Mayo Clinic Alumni magazine. You can read or download a PDF of the issue here.
Mayo Clinic alumni are entitled to the print version of the quarterly magazine. If you’re not receiving the magazine, register or log in to your online MCAA profile to make sure your address is correctly entered. Or contact the Alumni Association at mayoalumni@mayo.edu or 507-284-2317 for help.
Credit: Illustrations by Spooky Pooka