Transplant: Expanding the donor pool for lung transplants

When he got the call that a potential lung transplant was available in the fall of 2024, Bill Peterson and his wife, Joellen, were ready.
They got in the car to make the two-hour drive from their home in Eau Claire, Wisconsin, to Mayo Clinic in Rochester, Minnesota. It was snowing, but not heavily enough to deter them. As a man who had never had surgery before, much less a major, lifesaving one, Bill was somewhat anxious — but knew that transplant was his only option.
Upon arriving, Bill was admitted and prepped to go into surgery. After the procurement team assessed the donor lungs, transplant surgeon Sahar Saddoughi, M.D., Ph.D. (S ’19, TS ’21), Division of Thoracic Surgery at Mayo Clinic in Minnesota, came into Bill’s room. She had bad news: the lungs weren’t deemed good enough for transplant.
“So there I was on the bed getting ready to go into surgery and had to get dressed and go home,” says Bill.
Bill returned home to his oxygen tanks and largely homebound life. In 2022, he had been diagnosed with idiopathic pulmonary fibrosis and had lost weight, muscle and freedom in just a few years.
“The disease progressed to a point where the portable tank didn’t give me sufficient oxygen, so I had to lug around a big tank,” he says. “I couldn’t be very active and couldn’t do much of anything.”
A few weeks later, Bill got another call for a potential transplant, so he and Joellen again made the drive to Mayo Clinic — only to be told while in admissions that the lungs were again not transplantable. Bill and Joellen drove home, and Bill resumed his place on the transplant waiting list.
As of June 2025, there were over 900 people waiting for a lung transplant in the U.S. Because only about 20% to 30% of donor lungs meet the standard for transplant, there are never enough lungs to go around. This means that every year, patients die waiting for donor lungs or become too sick to receive a transplant — outcomes Dr. Saddoughi finds unacceptable.
So in addition to her clinical practice, Dr. Saddoughi researches ways to expand the donor pool and increase the number of lung transplants. She tests novel transplant therapies and procedures in large animal models, and her lab explores extended-criteria donors, lung rehabilitation and next-generation lungs.
“The availability of high-quality organs for lung transplant is insufficient in the U.S.,” says Cassie Kennedy, M.D. (I ’03, CMR ’04, CI ’06, THDC ’08, CTSA ’24), medical director of lung transplantation at the William J. von Liebig Center for Transplantation and Clinical
Regeneration at Mayo Clinic in Minnesota. “Science that makes ‘unusable’ organs transplantable will increase the number of transplants and save lives.”
In 2023, Dr. Saddoughi used her porcine lab to help clarify some of the controversy surrounding the use of normothermic regional perfusion for lungs — a contribution that would have direct relevance for Bill and could help many others like him.
“I’m excited about the type of research we do because we are able to translate from bench to bedside and directly change patients’ lives,” says Dr. Saddoughi.
WIDENING THE DONOR POOL
For a long time, transplants from deceased donors were largely limited to transplants resulting from donation after brain death (DBD). Meanwhile, the use of donation after circulatory death (DCD) made up a paltry percentage of total transplants. In 2000, there were just 206 total DCD organ transplants compared to 17,088 DBD transplants in the U.S.
One reason for this disparity had to do with the quality of the donor organs: DBD donors are placed on a ventilator to circulate oxygen and preserve the organs before transplant. For a DCD donor, all life-sustaining measures are stopped; patients then undergo circulatory arrest and asystole. This is followed by a declaration of death and a several minute waiting period before organ procurement — a time when vital organs can be exposed to warm ischemia.
In the case of lung transplant, ex vivo lung perfusion (EVLP) technology can help transplant teams assess whether questionable or marginal lungs — including those from DCD donors — are suitable for transplant. EVLP allows transplant teams to monitor and evaluate post-procurement donor lungs outside of the body for several hours.
In 2019, there were major advances in heart transplant that had big implications for lung transplant in the U.S. Two new technologies revitalized the idea of DCD heart
transplant: normothermic regional perfusion (NRP) and the Organ Care System (OCS), an ex-situ perfusion system known as “heart in a box.” Both reanimate the heart and allow medical teams to assess heart function before transplant.
NRP and OCS rapidly increased the use of DCD hearts and helped contribute to the rise of DCD organs in general. By 2024, DCD transplants made up 27% of all transplants.
But some in the lung transplantation field began to question NRP’s effect on lung graft function.
Marginal lungs can be sent to bioengineering facilities — such as the facility at Mayo Clinic in Florida — for assessment, and these centers were noticing that many post-NRP lungs sent to them were of poor quality, Dr. Saddoughi says. This brought up questions: Was some aspect of the procurement process injuring the lungs? Could the lungs be better protected during procurement?
To help verify whether NRP procurement was damaging lungs, Dr. Saddoughi turned to her porcine lab.
“I thought this was a question I could answer for myself through my research,” says Dr. Saddoughi.
Her team then established an NRP pig model. Eight pigs underwent circulatory arrest; four were randomized to NRP. The team followed a procedure similar to human NRP procurement: the NRP pigs were quickly placed on bypass for an hour with their head vessels clamped, allowing the heart to start beating again before procurement. As a control, the remaining four pigs went straight to procurement, with no NRP.
All lungs were then placed on EVLP for three hours to evaluate lung function, including oxygenation capacity and airway pressure. The team also looked for gross damage, took X-rays of the lungs, reviewed markers of inflammation, compared metabolic profiles and had an independent pathologist review the lung tissue. All these measures were comparable between the groups, and all lungs were deemed suitable for transplant.
“We found that in this very controlled situation, the lungs seemed to be functioning well,” says Dr. Saddoughi. “The outcome was that these lungs after NRP were still in good condition and should be considered for transplant.”
The study — along with her research looking at early national outcomes of DCD lungs and a meta-analysis comparing DCD and DBD short- and long-term transplant outcomes — provided the reassurance Dr. Saddoughi needed to proceed with these types of transplants in her operating room.
“My goal as a lung transplant surgeon is to continue to find ways to open up the donor pool for patients,” she says. “I was really comforted by the result. Because I believe in it, and now I can feel confident about that, as both a researcher and a surgeon.”
How it works: Normothermic regional perfusion
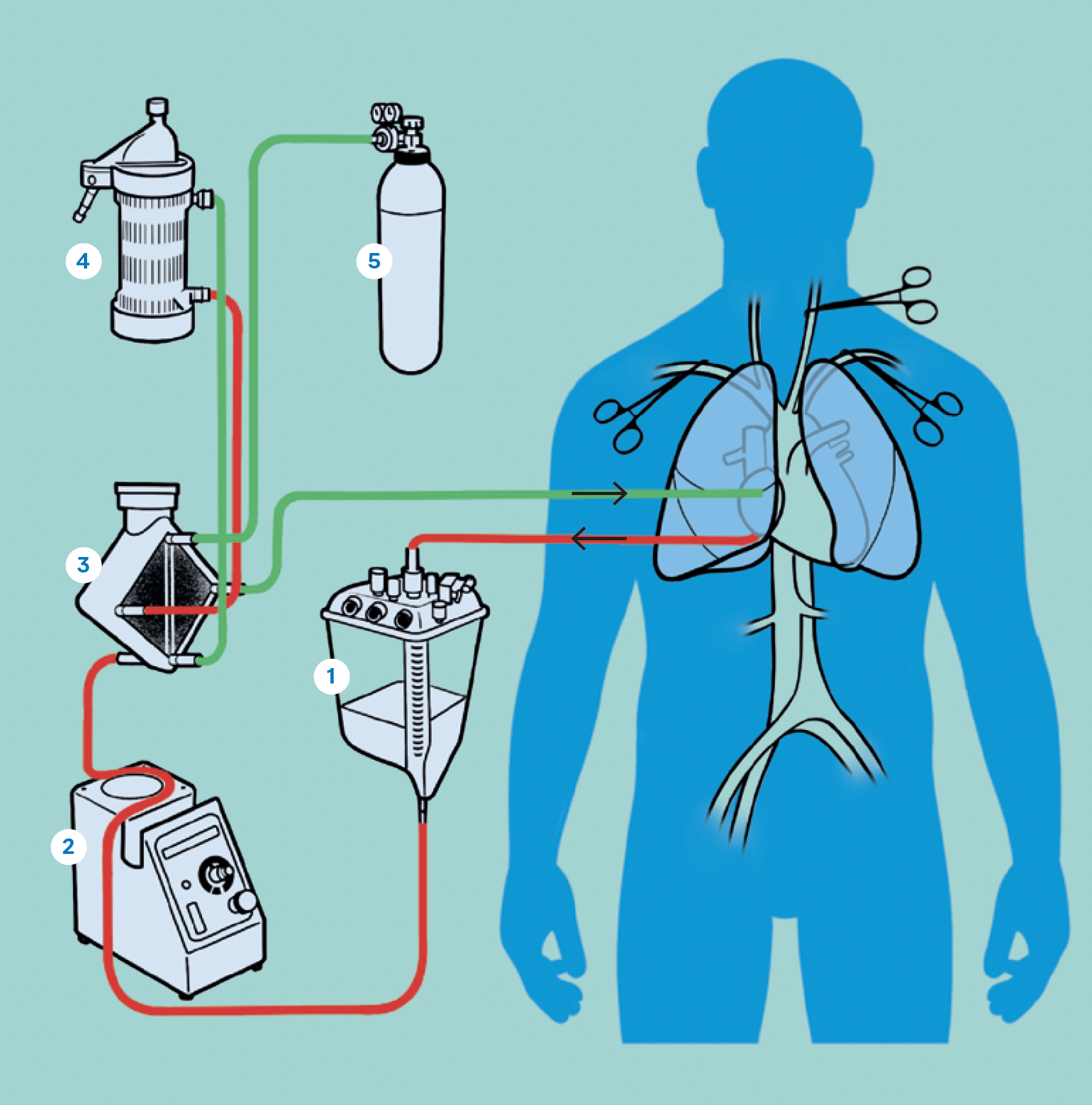
In NRP, the donor is declared dead after circulatory arrest and asystole. A surgical team then opens the sternum and inserts cannulas into the aorta and the right atrium of the heart, starting the donor on cardiopulmonary bypass. The heart restarts and circulates blood throughout the chest and abdomen, limiting organ ischemia. The vessels to the brain are clamped prior to starting cardiopulmonary bypass, ensuring no blood circulation to the brain. NRP continues for about an hour to revive and assess the organs before procurement.
- Reservoir
- Roller pump
- Membrane oxygenator
- Heat exchanger
- Gas mixer
THE BEST LUNGS FOR BILL
When Bill received his third call about a potential transplant in December 2024, he knew he may arrive in Rochester to be disappointed again. But his previous trips for a potential transplant had actually helped cement Bill and Joellen’s trust in the healthcare team.
“It gave us confidence that they’re looking out for us, and they wanted the best lungs for Bill,” says Joellen.
If the Mayo Clinic team believed donor lungs to be transplantable, Bill and Joellen were on board — and that confidence extended to DCD lungs. So on Dec. 5, 2024, Bill was successfully transplanted by Mayo Clinic surgeon Philip Spencer, M.D. (CS ’22), with NRP lungs from a DCD donor.
Recovery from lung transplant was intense, but Bill remains grateful for the lung function the transplant has given him and savors the fact that he’s free from the need for supplemental oxygen.
“I still have some work to do to build up my strength and stamina. But I know there’s an end result, which will be getting back to some degree of normalcy that I had,” Bill says.
Like most areas of science, the issue of NRP lungs is not settled. A 2024 American Association for Thoracic Surgery consensus statement says that there are limited data on the effect of thoraco-abdominal NRP on lung function. Dr. Saddoughi, a co-author of the statement, says the practice of NRP continues to evolve.
“There is a lot of uncertainty in lung transplant. There is no such thing as a perfect donor organ,” says Dr. Kennedy. “Science that reduces transplant providers’ uncertainty about an organ’s future performance in the lifesaving tasks of oxygenation and ventilation will improve recipient outcomes and organ utilization.”

LOOKING FORWARD
As Dr. Saddoughi looks forward, there are more research questions she wants to help answer, such as whether lung-derived exosomes can help mitigate ischemia-reperfusion injury in DCD transplant. She also wants to explore other areas of donor expansion like xenotransplantation, 3D-printed organs, and decellularized and recellularized lungs.
“I feel extremely fortunate to be part of the Mayo Clinic lung transplant team. The program has transformed significantly over the last several years due to the incredible teamwork and leadership. In 2024 alone, we were able to help 84 patients through the amazing gift of lung transplantation,” says Dr. Saddoughi. “My hope is to continue my research to ask important questions like: Can we take lungs that are not transplantable and find a way to make them transplantable?
“Ultimately, the goal is to continue to help more patients with end-stage lung disease breathe again.”
As for Bill, he exercises every day and takes regular walks with Joellen, extending the distance bit by bit. They’re working up to participate in an Eau Claire turkey trot with a fellow local double-lung transplant recipient they met in Rochester.
It’s hard for Bill to express what the transplant means to him — a challenge he faced directly when he wrote a letter to the donor family.
“Words can’t describe the gratitude I have, and how I feel about this generous gift, really a gift of life,” Bill says.
“Our daughter wrote a two-page letter just thanking them, because now she has her dad back and her kids will know their grandfather,” Joellen says.
READ THE ENTIRE BENCH TO BESIDE SERIES
This story appears in the latest issue of Mayo Clinic Alumni magazine. You can read or download a PDF of the issue here.
Mayo Clinic alumni are entitled to the print version of the quarterly magazine. If you’re not receiving the magazine, register or log in to your online MCAA profile to make sure your address is correctly entered. Or contact the Alumni Association at mayoalumni@mayo.edu or 507-284-2317 for help.
Credit: Illustrations by Spooky Pooka