Regenerative Medicine: Offering hope for drug-resistant seizures
Tabitha Wilson began having seizures at the age of 2. Fortunately, they were well controlled with medications — but that began to change during her senior year of high school.
“I was 17 years old sitting in history class when the seizure happened. They had to load me up in an ambulance in front of the whole school,” says Tabitha. “It was traumatizing. Something I will never forget.”
After that, her seizures worsened. Despite trying multiple medications and undergoing brain surgeries, her seizures became debilitating and dangerous.
“I fell down a flight of stairs, I’ve burned myself while cooking, I’ve completely blacked out and don’t know where I am,” she says. “I can’t drive, can’t cook or swim alone, I can’t take a bath, only a shower, and someone has to be in the house.”
Tabitha was desperate for relief for her drug-resistant epilepsy (DRE) — a condition she shares with about 15 million people worldwide.
“I’m willing to try everything and anything to get some sort of control over these seizures because I’ve been living with this for so long,” says Tabitha.
Clinicians like Sanjeet Grewal, M.D. (NS ’20), Department of Neurologic Surgery at Mayo Clinic in Florida, use every tool in their toolbox to help patients like Tabitha. But at some point, they run out of tools — a heartbreaking experience to witness in patients and frustrating as a physician, Dr. Grewal says. It’s a frustration that motivated him to pursue other solutions.
“I’ve been trained to be a surgeon, take care of people. But I’ve always had a scientific curiosity,” Dr. Grewal says. “As physicians, the moment we wonder why a patient’s outcome couldn’t be better is the moment science becomes indispensable — because every unanswered question is a doorway to improving care.”
In 2024, Tabitha became the first person in the world to receive a new potential therapy for her DRE as part of a phase 1 clinical trial led by Dr. Grewal at Mayo Clinic in Florida. The trial studies the use of deep brain stimulation (DBS) in conjunction with a stem cell implant in the thalamus.

A TEAM EFFORT
Current treatment options for DRE include invasive procedures such as brain resection or laser ablation therapy. While often effective, these surgical approaches carry the risk of side effects such as memory problems, motor deficits and speech impairment. And these options are not available to everyone with DRE.
“There’s a subset of patients whose seizures are coming from an area that we can’t necessarily resect or ablate,” Dr. Grewal says. “Those are the toughest ones for us to help. This trial is for our toughest patients.”
Dr. Grewal’s clinical trial uses stem cells to attempt a reparative, rather than destructive, approach.
“The paradigm shift here is that we’ve gone from ‘OK, here’s a problem in the brain, let’s take that problem out or let’s disrupt it’ to ‘Hey, there’s part of the brain that’s dysfunctional, let’s try to fix it,’” says Dr. Grewal. “So instead of removing or destroying tissue, we’re trying to regenerate it and repair it.”
DBS is an FDA-approved therapy for DRE and results in median seizure reduction of up to 70% after five years. But it’s uncommon for patients to become seizure-free with DBS — and that wasn’t good enough for Dr. Grewal.
“We thought, ‘Could we make DBS better by adding a regenerative component — adding these stem cells?’” he says.
To accomplish this, Dr. Grewal worked with a team of Florida colleagues, including neurologist William Tatum, D.O. (N ’09), and neurosurgeon Alfredo Quinones-Hinojosa, M.D. (NS ’16). Dr. Quinones-Hinojosa is also a William J. and Charles H. Mayo Professor and the James C. and Sarah K. Kennedy Dean of Research at Mayo Clinic in Florida.
The stem cells used in the clinical trial were produced at the Human Cellular Therapy Laboratory at Mayo Clinic in Florida under the leadership of Abba Zubair, M.D., Ph.D. (LABM ’03), a pioneer in cell therapy. Dr. Zubair is also the vice dean of Mayo Clinic Alix School of Medicine – Florida Campus.
“When it came to which cells to use, there’s so many different types,” says Dr. Grewal. “That’s where Dr. Zubair was very helpful.”
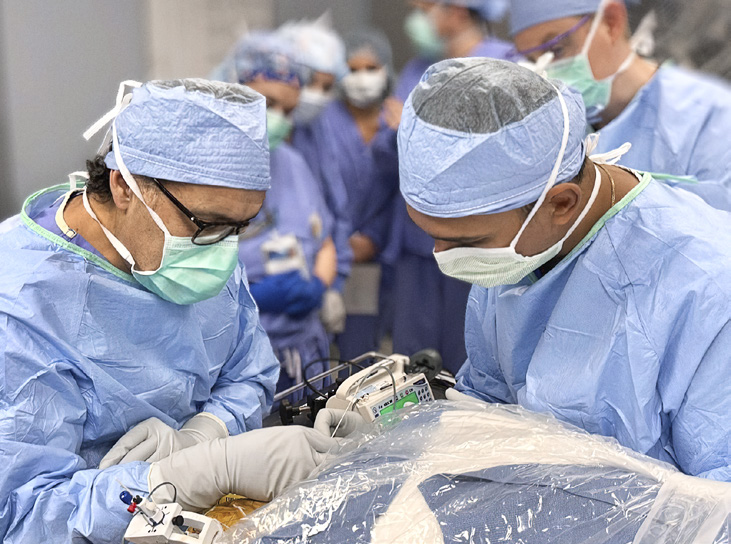
Dr. Zubair guided the team toward a type of adult stem cell known as mesenchymal stem cells (MSCs) derived from adipose tissue. When cultured, MSCs secrete a “milieu of factors,” Dr. Zubair says, including growth factors and cytokines, some of which are shown to be anti-inflammatory or induce regeneration. Inflammation is thought to contribute to the abnormal firing of neurons that occurs in epilepsy — but there are many possible mechanisms of the condition, Dr. Zubair says.
“Complex diseases require complex treatment,” says Dr. Zubair. “And the beauty of MSCs is they sense the environment and then, based on that, they respond to it and then secrete the right type and amount of these factors.
“So it’s not like we know what they secrete and could just inject those factors. It’s more of a cocktail and the cocktail depends on the situation at the exact time the cells interact with the environment. That makes it really unique and that’s why we think the MSCs are a good therapeutic agent in this setting.”
After deciding upon MSCs, Dr. Grewal’s team received funding from Mayo Clinic’s Center for Regenerative Biotherapeutics for research to ensure that DBS and MSC implants would not interfere with each other. The Practice Advancement Laboratory, under the guidance of Takahisa Kanekiyo, M.D., Ph.D. (NSCI ’10), Department of Neuroscience at Mayo Clinic in Florida, conducted in vitro experiments to show that electrical stimulation from DBS would not damage the MSCs, affect their properties or make them more migratory. With the help of senior research technologist Ralph Perkerson, the lab also concluded that MSCs would not interfere with DBS hardware in the brain or decrease its efficacy.
To ensure the cells were precisely implanted in the anterior nucleus of the thalamus, the team relied on the ultrahigh field 7-tesla (7T) MRI expertise of Erik Middlebrooks, M.D. (RD ’17), Division of Neuroradiology at Mayo Clinic in Florida.
This true team effort highlights the importance of collaboration, Dr. Grewal says.
“I’m inquisitive and want things to get better, but I also know my limitations,” says Dr. Grewal. “There are people who have trained for years and years to be scientists and I want to make sure I use their expertise for patients.”
Similarly, Dr. Zubair values the perspective that Dr. Grewal and other cross-specialty clinicians bring to his work — especially as it helps facilitate moving innovations from bench to bedside.
“I’m excited about science, but I’m even more excited about solving patients’ problems, giving them hope, developing new treatments,” Dr. Zubair says. “I’m always thrilled to see the product we generate in my lab taken out either to the floor or to the OR to be injected. It makes my day.”
How it works: MSC implantation
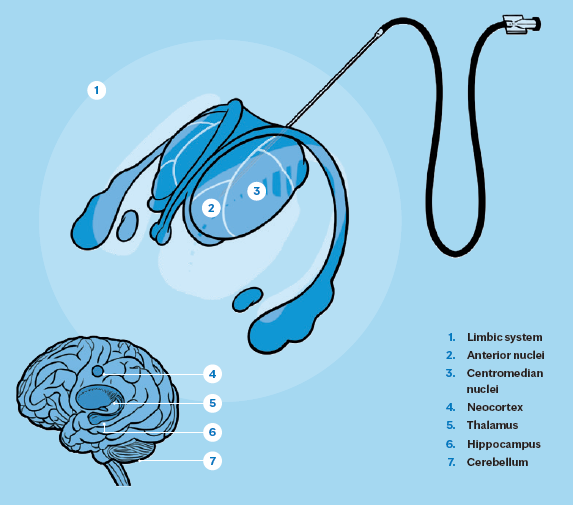
Seizures are the result of abnormal electrical brain activity; a person with epilepsy has a hyperexcitable brain. This could be due to an excess of excitatory electrical activity and/or a lack of inhibitory activity.
Mesenchymal stem cells (MSCs) are multipotent adult stem cells that can be derived from several sources, including adipose tissue, as in Dr. Grewal’s trial. MSCs secrete anti-inflammatory and neuroprotective molecules such as interleukin-10 (IL-10) cytokine, brain-derived neurotrophic factor (BDNF) protein and vascular endothelial growth factor (VEGF) into nearby brain tissue. The molecules could help mitigate potential contributors to epilepsy by reducing excitability of neurons and repairing damaged cells and tissue — such as by reducing scarring (gliosis), modulating excessive microglial pro-inflammatory activity, or slowing the loss of gamma-aminobutyric acid (GABA)ergic interneurons.
“We feel that the mechanism of action of MSCs is likely multimodal, through paracrine secretion of anti-inflammatory cytokines, secretion of tissue reparative factors such as BDNF, and potentially inducing proliferation, migration and differentiation of the endogenous neural stem cells,” Dr. Grewal says.
In this trial, Dr. Grewal’s team targeted the anterior nucleus of the thalamus (ANT) for cell implantation. The ANT is a critical hub within thalamocortical and limbic circuits involved in seizure propagation. Its selection was informed by prior success with deep brain stimulation (DBS) in this region, which has been shown to decrease network excitability. It’s hoped that MSCs in this location can similarly act synergistically with the DBS as a form of circuit-specific neuromodulation.
HOPE ON THE HORIZON
There is a long road ahead to determine whether this cell therapy in conjunction with DBS results in clinical improvement for patients with DRE, Dr. Grewal says. Though Tabitha has experienced improvement in her seizure management since the procedure, the initial study simply aims to confirm the safety and feasibility of the operation.
“We wanted to be very cautious initially,” says Dr. Grewal.
If proven safe, future steps in researching this therapy would be dose-escalation trials and possible bilateral implantation of the cells in the brain. In the meantime, researchers like Dr. Grewal and Dr. Zubair remain committed to investigating whether cell therapies could be the solution for the many patients like Tabitha.
“Our tools are getting better and better; cells and genetic therapies are getting more and more sophisticated. That’s allowing us to tackle some of these questions we haven’t been able to in the past,” says Dr. Grewal. “So, whether it’s wound healing, neurodegeneration, epilepsy or stroke, there are so many different studies going on investigating the potential of regenerative or reparative therapies.”
“We know these products sometimes don’t work, but we also do know they generate hope,” says Dr. Zubair. “Everybody can have that hope when they come to Mayo, that despite everything else failing, here is something new that can be tried in a safe way.”
READ THE ENTIRE BENCH TO BESIDE SERIES
This story appears in the latest issue of Mayo Clinic Alumni magazine. You can read or download a PDF of the issue here.
Mayo Clinic alumni are entitled to the print version of the quarterly magazine. If you’re not receiving the magazine, register or log in to your online MCAA profile to make sure your address is correctly entered. Or contact the Alumni Association at mayoalumni@mayo.edu or 507-284-2317 for help.
Credit: Illustrations by Spooky Pooka

